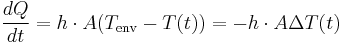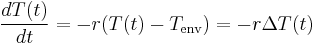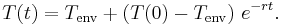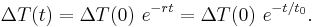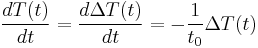Lumped capacitance model
A lumped capacitance model, also called lumped system analysis,[1] reduces a thermal system to a number of discrete “lumps” and assumes that the temperature difference inside each lump is negligible. This approximation is useful to simplify otherwise complex differential heat equations. It was developed as a mathematical analog of electrical capacitance, although it also includes thermal analogs of electrical resistance as well.
The lumped capacitance model is a common approximation in transient conduction, which may be used whenever heat conduction within an object is much faster than heat conduction across the boundary of the object. The method of approximation then suitably reduces one aspect of the transient conduction system (spacial temperature variation within the object) to a more mathematically tractable form (that is, it is assumed that the temperature within the object is completely uniform in space, although this spacially-uniform temperature value changes over time). The rising uniform temperature within the object or part of a system, can then be treated like a capacitative reservoir which absorbs heat until it reaches a steady thermal state in time (after which temperature does not change within it).
An early-discovered example of a lumped-capacitance system which exhibits mathematically simple behavior due to such physical simplifications, are systems which conform to Newton's law of cooling. This law simply states that the temperature of a hot (or cold) object progresses toward the temperature of its environment in a simple exponential fashion. Objects follow this law strictly only if the rate of heat conduction within them is much larger than the heat flow into or out of them. In such cases it makes sense to talk of a single "object temperature" at any given time (since there is no spatial temperature variation within the object) and also the uniform temperatures within the object allow its total thermal energy excess or deficit to vary proportionally to its surface temperature, thus setting up the Newton's law of cooling requirement that the rate of temperature decrease is proportional to difference between the object and the environment. This in turn leads to simple exponential heating or cooling behavior (see below for detail).
Contents |
Method
To determine the number of lumps the Biot number (Bi), a dimensionless parameter of the system, is used. Bi is defined as the ratio of the conductive heat resistance within the object to the convective heat transfer resistance across the object's boundary with a uniform bath of different temperature. When the thermal resistance to heat transferred into the object is larger than the resistance to heat being diffused completely within the object, the Biot number is less than 1. In this case, particularly for Biot numbers which are even smaller, the approximation of spatially uniform temperature within the object can begin to be used, since it can be presumed that heat transferred into the object has time to uniformly distribute itself, due to the lower resistance to doing so, as compared with the resistance to heat entering the object.
If the Biot number is less than 0.1 for a solid object, then the entire material will be nearly the same temperature with the dominant temperature difference will be at the surface. It may be regarded as being "thermally thin". The Biot number must generally be less than 0.1 for usefully accurate approximation and heat transfer analysis. The mathematical solution to the lumped system approximation gives Newton's law of cooling.
A Biot number greater than 0.1 (a "thermally thick" substance) indicates that one cannot make this assumption, and more complicated heat transfer equations for "transient heat conduction" will be required to describe the time-varying and non-spatially-uniform temperature field within the material body.
The single capacitance approach can be expanded to involve many resistive and capacitive elements, with Bi < 0.1 for each lump. As the Biot number is calculated based upon a characteristic length of the system, the system can often be broken into a sufficient number of sections, or lumps, so that the Biot number is acceptably small.
Some characteristic lengths of thermal systems are:
For arbitrary shapes, it may be useful to consider the characteristic length to be volume / surface area.
Thermal purely resistive circuits
A useful concept used in heat transfer applications once the condition of steady state heat conduction has been reached, is the representation of thermal transfer by what is known as thermal circuits. A thermal circuit is the representation of the resistance to heat flow in each element of a circuit, as though it were an electrical resistor. The heat transferred is analogous to the electrical current and the thermal resistance is analogous to the electrical resistor. The values of the thermal resistance for the different modes of heat transfer are then calculated as the denominators of the developed equations. The thermal resistances of the different modes of heat transfer are used in analyzing combined modes of heat transfer. The lack of "capacitative" elements in the following purely resistive example, means that no section of the circuit is absorbing energy or changing in distribution of temperature. This is equivalent to demanding that a state of steady state heat conduction (or transfer, as in radiation) has already been established.
The equations describing the three heat transfer modes and their thermal resistances in steady state conditions, as discussed previously, are summarized in the table below:
| Transfer Mode | Rate of Heat Transfer | Thermal Resistance |
|---|---|---|
| Conduction | 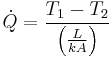 |
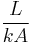 |
| Convection | 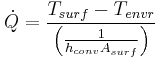 |
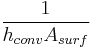 |
| Radiation | 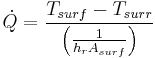 |
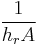 , where , where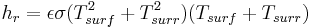 |
In cases where there is heat transfer through different media (for example, through a composite material), the equivalent resistance is the sum of the resistances of the components that make up the composite. Likely, in cases where there are different heat transfer modes, the total resistance is the sum of the resistances of the different modes. Using the thermal circuit concept, the amount of heat transferred through any medium is the quotient of the temperature change and the total thermal resistance of the medium.
As an example, consider a composite wall of cross-sectional area 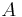 . The composite is made of an
. The composite is made of an 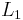 long cement plaster with a thermal coefficient
long cement plaster with a thermal coefficient 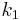 and
and 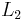 long paper faced fiber glass, with thermal coefficient
long paper faced fiber glass, with thermal coefficient 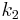 . The left surface of the wall is at
. The left surface of the wall is at 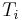 and exposed to air with a convective coefficient of
and exposed to air with a convective coefficient of 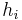 . The right surface of the wall is at
. The right surface of the wall is at 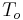 and exposed to air with convective coefficient
and exposed to air with convective coefficient 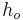 .
.
Using the thermal resistance concept heat flow through the composite is as follows:
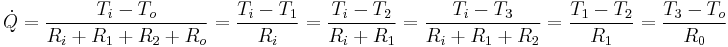
where
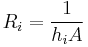 ,
, 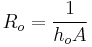 ,
, 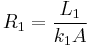 , and
, and 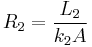
Newton's law of cooling: example of a thermal circuit with one resistive and one capacitative element
Newton's law of cooling describes many situations in which an object has a large thermal capacity and large conductivity, and is suddenly immersed in a uniform bath which conducts heat relatively poorly. This law stated in non-mathematical form is that the rate of heat loss of a body is proportional to the difference in temperatures between the body and its surroundings. (see below for the equivalent mathematical equation). For the law to be correct, the temperatures at all points inside the body must be approximately the same at each time point, including the temperature at its surface. Thus, the temperature difference between the body and surroundings does not depend on which part of the body is chosen, since all parts of the body have effectively the same temperature. In these situations, the material of the body does not act to "insulate" other parts of the body from heat flow, and all of the significant insulation (or "thermal resistance") controlling the rate of heat flow in the situation resides in the area of contact between the body and its surroundings. Across this boundary, the temperature-value jumps in a discontinuous fashion.
In such situations, heat can be transferred from the exterior to the interior of a body, across the insulating boundary, by convection, conduction, or diffusion, so long as the boundary serves as a relatively poor conductor with regard to the object's interior. The presence of a physical insulator is not required, so long as the process which serves to pass heat across the boundary is "slow" in comparison to the conductive transfer of heat inside the body (or inside the region of interest—the "lump" described in the introduction).
In such a situation, the object acts as the "capacitative" circuit element, and the resistance of the thermal contact at the boundary acts as the (single) thermal resistor. In electrical circuits, such a combination would charge or discharge toward the input voltage, according to a simple exponential law in time. In the thermal circuit, this configuration results in the same behavior in temperature: an exponential approach of the object temperature to the bath temperature.
Newton's law is mathematically stated by the simple first-order differential equation:
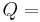 Thermal energy in joules
Thermal energy in joules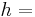 Heat transfer coefficient
Heat transfer coefficient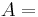 Surface area of the heat being transferred
Surface area of the heat being transferred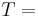 Temperature of the object's surface and interior (since these are the same in this approximation)
Temperature of the object's surface and interior (since these are the same in this approximation)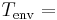 Temperature of the environment
Temperature of the environment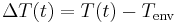 is the time-dependent thermal gradient between environment and object
is the time-dependent thermal gradient between environment and object
Putting heat transfers into this form is sometimes not a very good approximation, depending on ratios of heat conductances in the system. If the differences are not large, an accurate formulation of heat transfers in the system may require analysis of heat flow based on the (transient) heat transfer equation in nonhomogeneous, or poorly conductive mediums.
Solution in terms of object heat capacity
If the entire body is treated as lumped capacitance heat reservoir, with total heat content which is proportional to simple total heat capacity 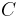 , and
, and 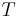 , the temperature of the body, or
, the temperature of the body, or 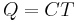 . It is expected that the system will experience exponential decay with time in the temperature of a body.
. It is expected that the system will experience exponential decay with time in the temperature of a body.
From the definition of heat capacity 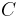 comes the relation
comes the relation 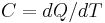 . Differentiating this equation with regard to time gives the identity (valid so long as temperatures in the object are uniform at any given time):
. Differentiating this equation with regard to time gives the identity (valid so long as temperatures in the object are uniform at any given time): 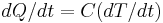 . This expression may be used to replace
. This expression may be used to replace 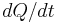 in the first equation which begins this section, above. Then, if
in the first equation which begins this section, above. Then, if 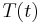 is the temperature of such a body at time
is the temperature of such a body at time 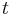 , and
, and 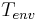 is the temperature of the environment around the body:
is the temperature of the environment around the body:
where
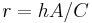 is a positive constant characteristic of the system, which must be in units of
is a positive constant characteristic of the system, which must be in units of 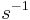 , and is therefore sometimes expressed in terms of a characteristic time constant
, and is therefore sometimes expressed in terms of a characteristic time constant 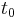 given by:
given by: 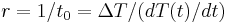 . Thus, in thermal systems,
. Thus, in thermal systems, 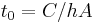 . (The total heat capacity
. (The total heat capacity 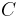 of a system may be further represented by its mass-specific heat capacity
of a system may be further represented by its mass-specific heat capacity 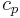 multiplied by its mass
multiplied by its mass 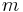 , so that the time constant
, so that the time constant 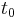 is also given by
is also given by 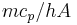 ).
).
The solution of this differential equation, by standard methods of integration and substitution of boundary conditions, gives:
If:
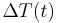 is defined as :
is defined as : 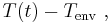 where
where 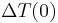 is the initial temperature difference at time 0,
is the initial temperature difference at time 0,
then the Newtonian solution is written as:
This same solution is almost immediately apparent if the initial differential equation is written in terms of 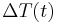 , as the single function to be solved for. '
, as the single function to be solved for. '
Applications
This mode of analysis has been applied to forensic sciences to analyze the time of death of humans. Also it can be applied to HVAC (heating, ventilating and air-conditioning, or building climate control), to ensure more nearly instantaneous effects of a change in comfort level setting.[2]